Powering the Immune System to Transform Lives
Selected publications
Science in the Spotlight
A Phase 2 Trial of Tobevibart Plus Elebsiran in Hepatitis Delta
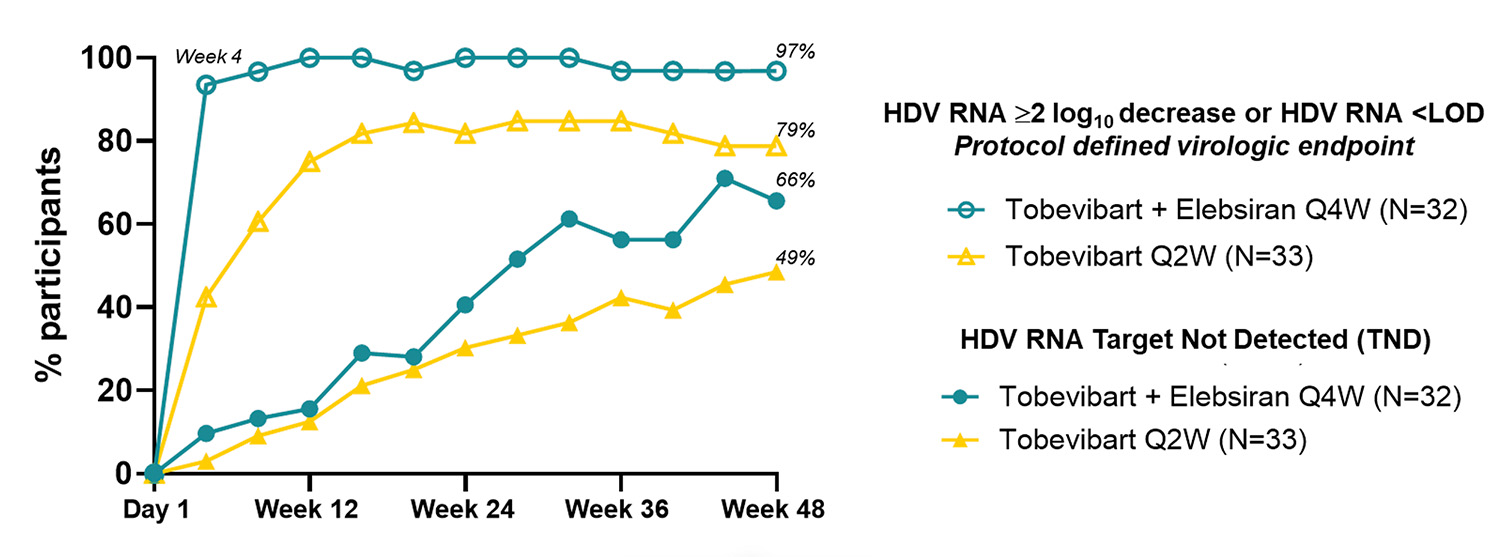
This New England Journal of Medicine manuscript describes the efficacy and safety results of Vir Biotechnology’s SOLSTICE Phase 2 clinical trial evaluating the combination of tobevibart and elebsiran for the treatment of chronic hepatitis delta.
The data show that the majority of participants (66%; 21/32) receiving a monthly dose of the combination achieved undetectable levels of hepatitis delta virus after 48 weeks of treatment (measured as hepatitis delta virus RNA target not detected). Additionally, approximately 90% of participants achieved reductions in hepatitis B surface antigen (HBsAg), which suggests that the fundamental mechanism that the hepatitis delta virus needs to replicate may be inhibited. The combination was well-tolerated, with no grade 3 or higher treatment-related adverse events and no treatment-related discontinuations.
The combination of tobevibart and elebsiran was selected for continued development based on its overall efficacy and safety profile. It is currently being evaluated in Vir Biotechnology’s ECLIPSE registrational program for chronic hepatitis delta.
Literature Archive
A Phase 2 trial of tobevibart plus elebsiran in hepatitis DNew England Journal of Medicine, 2025
Infectious Diseases Chronic Hepatitis Delta
PRO-XTEN® Oncology
Discovery and Engineering Infectious Diseases HIV
Discovery and Engineering Infectious Diseases Chronic Hepatitis Delta
Infectious Diseases Chronic Hepatitis Delta
Large language models for science and medicineEuropean Journal of Clinical Investigation, 2024
Artificial Intelligence
Infectious Diseases
A potent pan-sarbecovirus neutralizing antibody resilient to epitope diversificationCell, 2024
Discovery and Engineering Artificial Intelligence Infectious Diseases
PRO-XTEN® Oncology
Pandemic preparedness strategies must go beyond vaccinesScience Translation Medicine, 2023
Infectious Diseases
A pan-influenza antibody inhibiting neuraminidase via receptor mimicryNature, 2023
Discovery and Engineering Infectious Diseases
Neutralization, effector function and immune imprinting of omicron variantsNature, 2023
Discovery and Engineering Infectious Diseases
Discovery and Engineering Infectious Diseases Chronic Hepatitis Delta
Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagementScience, 2022
Infectious Diseases
Artificial Intelligence Infectious Diseases
Infectious Diseases
Tackling COVID-19 with neutralizing monoclonal antibodiesCell, 2021
Discovery and Engineering Infectious Diseases
Infectious Diseases
Infectious Diseases Chronic Hepatitis Delta
Broadly neutralizing antibodies overcome SARS-CoV-2 omicron antigenic shiftNature, 2021
Discovery and Engineering Infectious Diseases
Developing therapeutic monoclonal antibodies at pandemic paceNature Biotechnology, 2020
Discovery and Engineering Infectious Diseases
Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibodyNature, 2020
Discovery and Engineering Infectious Diseases
Fc-optimized antibodies elicit CD8 immunity to viral respiratory infectionNature, 2020
Discovery and Engineering Infectious Diseases
Discovery and Engineering Infectious Diseases
HIV: human immunodeficiency virus
Vir Biotechnology has exclusive rights to the PRO-XTEN® masking platform for oncology and infectious disease. PRO-XTEN® is a trademark of Amunix Pharmaceuticals, Inc., a Sanofi company.
